Heat Treatment

Heat treatment of metals is a commonplace, but often complex set of procedures aimed at enhancing mechanical properties. Why is heat treatment necessary, though, and how is it done? Read on to find out.
Contents
What is Heat Treating?
Heat treatment entails heating and cooling metal, altering its mechanical properties. Note that metal should never reach the molten state during the heat treatment process. Under this banner, techniques included are annealing, case hardening, precipitation strengthening, tempering, carburizing, normalizing, and quenching.
Heat treatment unlocks specific mechanical properties in metals that aren’t there in the metal’s original state. When done correctly, heat treatment enhances a metal’s strength and durability, although this usually comes at the cost of flexibility. When heat treatment processes go wrong, the metal’s mechanical integrity could be compromised. This is what happens during a building fire – the reinforcing metal is heated and cooled in an uncontrolled fashion. It should be replaced when rebuilding the structure.
Heat Treatment Process
The heat treatment process varies according to the aims you would like to achieve. However, there are specific commonalities to all controlled heat treatment processes: heating, soaking, and cooling. Note that it alloys are usually heat-treated since mechanical changes aren’t that common in pure metals.
The 3 Stages of Heat Treatment
1. Heating:
Metallic alloys in the solid-state are either a solid solution, a mechanical mixture, or a combination of these two. A mechanical mixture is comparable to concrete: portions of each metal are visible under a microscope, with a matrix of the one metal holding pieces of the other metal in place.
In contrast, a solid solution appears as the name suggests. The alloy’s metals are combined at the molecular level and are indistinguishable from each other.
Heating metal causes changes on a molecular level. Usually, a metal that is a mechanical mixture at room temperature will become a solid solution when heated sufficiently. Along with this, the metal’s grains size changes, affecting mechanical properties.
2. Soaking:
Soaking involves keeping your metallic alloy at the desired temperature until the entire structure is evenly heated. The larger the object in question, the longer the soaking period.
3. Cooling:
Cooling leads to mechanical changes in metals. These are predictable and depend on the cooling rate and the specifics of the metallic alloy in question. As with all other parts of the heat treatment process, tight control over the cooling process produces specific mechanical characteristics in the metal.
Types of Microstructures in Ferrous Metals
It is useful to understand the microstructure of ferrous metals before delving into the details of heat treatment processes. On a microscopic level, ferrous alloys usually comprise of a granular microstructure that differs according to the alloying elements used, and the temperature to which the metal is heated. Each of these microstructures has specific properties, rendering it suitable or unsuitable for particular applications. Heat treatment processes aim to alter the microscopic structure to the form best suited to the metal component’s intended end-use.
- Ferrite:
Ferrite forms a needle-like granular structure that is soft, ductile, and generally not tough.
- Austenite:
Austenite is a solid solution of ferrite and other alloying elements. It serves as the parent phase for most microstructural transformations, and it thus viewed as extremely useful and versatile. Austenite tends to be soft and ductile and is generally not stable at room temperature. Exceptions to this rule are high-carbon steels and its alloys.
Lack of control during the heat treatment process leads to residual austenite at room temperature, negatively affecting the mechanical properties of the component produced.
- Graphite and Cementite
Graphite is the stable form of carbon in ferrous metals, while cementite is its metastable cousin. Graphite is generally present in cast iron and can take various microstructural forms, depending on the working conditions of the metal. Usually, it is present as nodules suspended in the alloy.
- Austenitic Transformation Products
Carbon and alloy steels that are hot worked, tend to have an austenitic microstructure. Heat treatment transforms this microstructure into martensite, bainite, ferrite, and pearlite.
At the lower critical temperature, austenite transforms into ferrite and cementite. Below the critical temperature, pearlite forms. This is a metastable aggregate of ferrite and cementite formed into a lamellar pattern.
At lower temperatures, bainite forms, while martensite forms at even lower temperatures. The formation of these microstructures depends on the cooling rate.
While pearlite has a feathery microstructure, bainite is more cubic in nature, while martensite forms in lumps. Martensite is the softest and most ductile of these components.
Types of Heat Treatment Techniques
Hardening
Hardening is a common heat treatment technique for ferrous metals (metals containing iron). It involves slowly heating the metal to the desired temperature and cooling it rapidly through quenching (discussed later). At the microscopic level, the metal’s grain structure is predictable. This structure depends on the alloy’s constituents and its temperature. Each structural type has specific mechanical properties, such as hardness, tensile strength, and flexibility.
Heating the alloy to the desired temperature leads to the formation of the desired microscopic structure. Rapid cooling (quenching) “freezes” this grains structure, since the metal is now too cold to revert to the microscopic structure you would expect at the colder temperature. Grain structures found at a higher temperature usually offer greater strength and hardness to the metal, but it also leads to brittleness. A general rule of thumb is that harder, stronger metals tend to be more brittle.
Quenching
Quenching involves rapid cooling, usually in a cooling medium such as oil, water, air, or brine (a saltwater solution). Some metals warp and crack during quenching, especially when using the wrong quenching medium, so it is essential to match the quenching medium to the metal at hand.
Rapid cooling occurs in water or brine, while oil offers a slower rate of cooling. Air provides the slowest cooling rate. As a rule of thumb, we would quench carbon steels in water or brine, and alloy steels in oil.
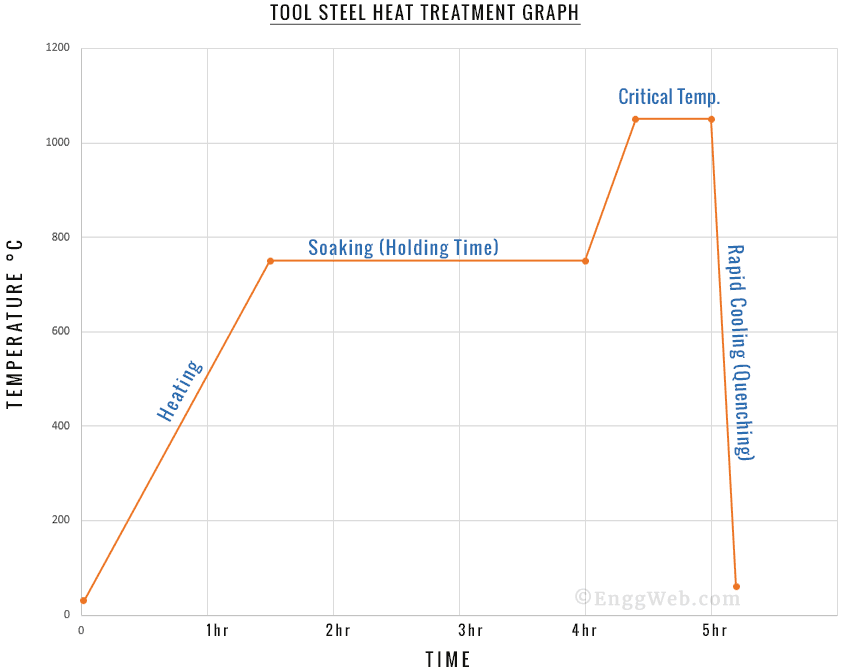
* Soaking time depends on the thickness and the shape of the workpiece.
During quenching, heated metal is submerged into the quenching medium to cool it rapidly. Once at the desired temperature, the metal is removed from the quenching medium and cooled to room temperature, usually in the air. Here, the metal’s microscopic structure gets “frozen,” maintaining the desired mechanical properties at room temperature.
Quenching causes high internal stresses in the metal. Here, the metal is extremely hard but far too brittle for practical use – a condition rectified by tempering (more on this later).
Note that metals quenched in brine should be thoroughly rinsed and cleaned after quenching since the salt in brine could cause rusting.
Tempering
Tempering relieves internal stresses formed during the quenching process. Here, metal (usually steel) is heated to a predetermined temperature and left to cool. This heating process allows the metal’s grain structure to rearrange slightly to relieve the stress, but not enough to cause excessive strength loss. Tempering does cause slight strength loss in the metal, but this is offset by the decrease in brittleness, rendering the metal more suitable for practical use.
The rate of cooling during the tempering process generally does not affect the metal’s mechanical properties. For this reason, tempered metal is usually cooled in still air, since this is cheaper and easier than using a specific cooling medium.
Surface Hardening
- Case hardening – Carburizing
Some applications require tough and wear-resistant parts, such as gears, chisels, and cylinder sleeves. Here, case hardening is an ideal heat treatment process, usually applied to low-carbon steel. Both carbon steel and low-carbon allow steel are suited to this process, which takes several hours to complete.
During carburizing, the metal part in question is heated to a predetermined temperature in the presence of the hardening component – carbon, in this case. The carbon could be in solid, liquid, or gas form, all of which decomposes and deposits on the metal in question.
This layer carbon layer remains on the part’s surface through rapid cooling, forming a tough, wear-resistant surface layer. The carbon deposit changes the surface layer’s chemistry, hence the extreme wear-resistance. The inner layers remain unaffected and are thus softer than the outside layer, but still extremely tough.
- Cyanide hardening
Cyanide hardening is a fast (half an hour from start to completion), efficient case hardening process that produces components with a far greater surface hardening than achieved through carburizing. It does, however, involve cyanide, which is lethal.
Here, we dip the part to be hardened into a heated cyanide bath and allow it to soak for a predetermined time. Upon removal, this part is quenched and rinsed to remove residual cyanide. The resulting cyanide shell is much thinner than the surface layer formed through carburizing, making this process ideal for small, precision parts.
- Nitriding
Nitriding differs from other surface hardening processes in that it requires no quenching, reducing the risk of warping. Here, parts are heat-treated and tempered before case hardening. It is important to note that nitriding produces the hardest, most wear-resistant surface of all case hardening processes.
For nitriding, we use a furnace containing ammonia gas. This ammonia gas diffuses into the metal’s surface, causing the microstructure to harden considerably. We generally use the nitriding process for parts manufactured from high-carbon, low-alloy steel. However, other alloys are also suited to this process, including medium and high-carbon steels, titanium, aluminum, and molybdenum.
- Carbonitriding
Carbonitriding is a combination of carburizing and nitriding. This process is suitable for parts made of most types of steel. Since it occurs at a lower temperature than carburizing, it reduces the distortion risk associated with rapid quenching. Carbonitriding produces a wear-resistant surface harder than that produces through carburizing, but softer than with nitriding.
During carbonitriding, metals parts are placed in a furnace saturated with a gaseous mixture of carbon and nitrogen. These gasses diffuse into the metal’s surface, causing it to harden. Nitrogen stabilizes the granular structure, allowing for further wear resistance.
After diffusion, the part is quenched. Since this process doesn’t require extremely high quenching rates, oil is usually used as a quenching medium, reducing the risk of warping.
Decarburization
Decarburization removes a metal’s carbon surface layer through surface degradation. In some cases, this is intentional, while in others, it is an unintended side effect of a heat treatment process gone wrong. To avoid decarburization during heat treatment processes, these processes must be carried out in an inert atmosphere.
When heating a metal above its lower critical temperature, the surface carbon layer diffuses into the atmosphere through reaction with oxygen and hydrogen. This process leaves the metal’s surface soft and with a low wear-resistance, precisely the opposite of the aim of carburization. This could also occur through a chemical reaction when exposing the carburized part to harmful chemical compounds.
Another side effect of decarburization is reducing fatigue resistance. When decarburized metals crack, the crack growth and wear rate are far higher than for non-carburized steel.
In cases where carburization was excessive, some decarburization could rectify the error.
Normalizing
Manufacturing processes, such as machining, forging, and welding, produce internal stresses in metals. These stresses could reduce the wear- and impact-resistance of the metals, and should thus be remedied, hence the normalizing process.
Normalizing relieves internal stresses in ferrous steel, increasing the metal’s toughness and its lifespan in high-wear environments. Normalizing is carried out before hardening and tempering since it helps develop the desired hardness in the metal if the hardening process is carried out correctly. During this process, we heat the metal to a higher level than that used for hardening and annealing (more on that later). The metal is soaked at this temperature until it is uniformly heated and then allowed to cool in still air. There is no quenching in this process since this introduces the risk of warping.
Normalized metals are softer and easier to machine, and generally stronger and harder than annealed metals.
Annealing
Annealing is similar to normalizing in that the metal is heated to above the critical range, soaked, and cooled slowly. The benefits of annealing are also similar to that of normalizing: removal of internal stresses, resulting in a softer, easily machined metal.
Annealing refines the metal’s grain structure, resulting in a more ductile metal.
Annealing differs from normalizing in that it remains at high temperature for a predetermined time, not necessarily long enough for it to be heated uniformly. Cooling rates also vary greatly. Sometimes, the metal is allowed to air cool, while other processes require packing or surface cooling. Packing involves covering the metal in the sand, ash, or other similar, inert substances that slow the cooling rate. During furnace cooling, the furnace is switched off while the metal remains inside the furnace. These two would then cool together, resulting in a prolonged cooling process.
Stress-Relieving
Stress-relieving is similar to normalizing and annealing in that the metal is heated to relieve internal stresses, and then cooled slowly. Here, the heating temperature is adequate to remove unwanted internal tensions and no higher than that. Usually, this is a far lower temperature than that used for annealing and normalizing. After heating, slow cooling prevents any new stresses from forming.
Specialty Heat Treatment Methods
Vacuum Hardening
Vacuum hardening increases steel’s oxidation resistance while providing low distortion. Here, steel is heated through convection in the absence of oxygen in a furnace saturated with nitrogen. The vacuum inside the furnace allows nitrogen to diffuse throughout the metal’s entire cross-section, resulting in a part that is of superior strength and hardness. Since the nitrogen-enriched atmosphere is inert, vacuum hardening prevents decarburization as well as carburization.
During vacuum hardening, the cooling rate is also precisely controlled, minimizing the risk of warping and other dimensional changes.
Flame Hardening
Flame hardening is a type of surface hardening. Here, metal components are heated locally using an oxyacetylene flame, after which it is immediately quenched with a stream of cold water. Since the metal is heated locally, the base metal assists in cooling since that is usually still at room temperature.
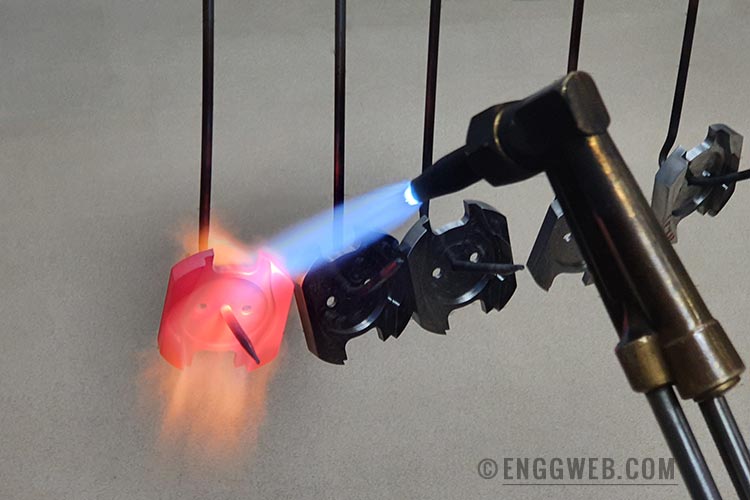
Flame hardening produces a hardened surface that is wear-resistant and tough. Simultaneously, the metal’s interior regions remain in their original state – usually tough, but soft in comparison.
This heat treatment process could be carried out manually, or through using automatic machinery. In either case, it requires close monitoring, since the metal’s temperature is determined visually. Overheating the surface area could lead to warping and produce undesired mechanical properties. Conversely, insufficient heating would not produce the desired surface hardness.
Precipitation Hardening (Aging)
Precipitation hardening, or aging, takes place at relatively low temperatures over an extended time.
Some alloys have non-uniform dispersion of their chemical components. This situation is usually as a result of rapid quenching, where the various components were “frozen” in place. Through aging, these chemical constituents disperse or precipitate, forming a uniform solid solution throughout the metal. Aging improves an alloy’s strength and other mechanical properties.
While some metals age naturally, at room temperature, others require an elevated temperature or artificial aging. The time and temperature necessary for aging differ according to the metal and its initial microstructure.
Austempering
Austempering improves ductility and strength and reduces distortion. Here, metal is submerged in a molten salt bath for an extended time. The aim is to produce a bainite microstructure, which tends to be relatively shock-resistant.
During the austempering process, high carbon steel is heated to within the austenitic range of 790 – 915°C (145 – 1675°F), hence the name. This steel is then quenched in a bath of molten salt, maintained at a constant temperature between 260 – 370°C (500 – 700°F). In this bath, which could contain oil instead of molten salt, the metal soaks until its microstructure completely transforms to bainite, after which it cools to room temperature.
Marquenching
Marquenching, a form of interrupted quenching, reduces distortion, cracking, and residual stress-build-up within steel components. This process is typical for complex parts. Here, the geometry, weight distribution, and sectional changes would lead to increased thermal stress formation.
In this process, tempering procedures occur as usual, but the metal is only quenched from an austenitizing temperature to one above the martensitic range. To this end, the metal could be quenched in a hot fluid medium until reaching a uniform temperature throughout the structure. Alternatively, we could slow the cooling rate to decrease the temperature difference between the metal’s surface and interior.
Deep Freeze
Deep freezing, or cryogenics, is a form of heat treatment were steel is cooled to somewhere between -75 and -185°C (-103 and -301°F). Usually, a martensitic microstructure is desired in heat-treated steels. This is far more ductile than the brittle, dimensionally unstable austenitic microstructure encountered at the normal hardening temperatures.
Martensite forms below a specific temperature, which depends on the type of steel and its alloying components. Most kinds of steel transform completely to martensite at room temperature. However, high carbon steel and high alloy steels retain a proportion of austenite at room temperature, which reduces the metal’s mechanical properties. Here, a lower temperature is needed to eliminate the austenitic microstructure, hence the extreme cooling of cryogenics.
To achieve this, metals are subjected to below-zero temperatures through the use of liquid nitrogen and soaked at this temperature until the entire microstructure transforms to martensite.
Heat Treating Stainless Steel
Heat treatment of stainless steels is potentially tricky. While heat treatment improves hardness, toughness, ductility, and overall strength, this often comes at the cost of corrosion resistance. Since stainless steels are, by nature, deemed as corrosion-resistant, this poses a challenge to the person tasked with heat treating these components.
For this reason, stainless steel heat treatment is carried out by experts who carefully match the intended use of each component to the heat treatment process. This also means that some forms of heat treatment aren’t suitable for stainless steel – this, again, depends on the intended use of the component.
Effects and Advantages of Heat Treatment
During heat treatment, the metal’s microstructure is altered and internal stresses relieved, resulting in a metal that is generally stronger, more ductile, and more crack-resistant. Ferrous metals, as-cast, are usually extremely hard, but brittle since they mostly comprise an austenite microstructure. We aim for different microstructures depending on the intended end-use of the metal since they have different mechanical properties. Heat treatment allows the formation of this specific microstructure, producing a component fit for its intended use.
Heat treatment also remedies errors from manufacturing. In some cases, temperature control during the manufacturing process goes awry, leading to the formation of internal stresses, incorrect microstructures, and other undesired consequences. Correct application of heat treatment processes remedies this.
Heat Treating Non-Ferrous Metals
Non-ferrous metals can receive heat treatment in one of two forms: annealing and solution heat treatment.
Annealing for non-ferrous metals is similar to that applied to ferrous metals, consisting of heating, soaking, and cooling. The peak temperature and method of cooling depend on the metal in question and its intended end-use. Most non-ferrous metals become increasingly brittle and hard through cold-working processes, and, at times, also after solution heat treatment. The annealing process removed the effects of this, improving the machining and working qualities of the metal.
Solution heat treatment improves the tensile strength of non-ferrous alloys. Here, heating the metal to a predetermined setpoint allows the alloy components to enter a solid-solution state. Rapid quenching “freezes” this microstructure, allowing the alloy to retain these new desired mechanical properties at room temperature.
Aluminum
Aluminum alloys are heat treated through solution heat treatment and annealing. During the solution heat treatment process, the alloy is heated to 527°C (980°F) and held there for an hour. After this, the metal is quenched in water to retain the desired microstructure. Annealing takes a bit longer and requires lower temperatures. Here, the metal is heated to between 163 and 204°C (325 and 400°F) and held there for one to eight hours. The lower the temperature, the longer the time required.
Copper
Copper and its alloys are usually heat treated through annealing and solution heat treatment. It is also suitable for stress relief heat treatment, similar to that of ferrous alloys. All of these methods reduce stresses formed during cold-working processes, improving the alloy’s ductility and strength.
Brass
Brass becomes hard and brittle during cold-working. To eliminate these adverse effects, soft-annealing is administered to the final product. Here, brass is heated to between 425 and 650°C (797 and 1202°F) and cooled slowly.
Titanium
In addition to annealing and solution heat treatment, titanium alloys are also suited to stress relief heat treatment and aging. All of these processes optimize the alloy’s mechanical properties, increasing fracture toughness, fatigue strength, high-temperature creep strength, and resistance to chemical attack. Simultaneously, we decrease the risk of distortion.
Summary
Heat treatment occurs in many different forms, varying depending on the metal and its intended end-use. When administered correctly, heat treatment improves a metal’s mechanical properties, increasing ductility and strength, reducing brittleness, and generally rendering the metal fit for its purpose.
FAQ (Frequently Asked Questions)
Why is heat treatment required?
The primary purpose of heat treatment is to harden or soften metals. It also eliminates, or at least reduces, adverse effects of other processes carried out on the metal in question. Most significantly, heat treatment reduces brittleness, leading to increased toughness and ductility.
Does heating metal make it weaker?
While heat treatment slightly reduces mechanical strength in some cases, it also reduces brittleness. The result is a metal that is far tougher and less prone to warping, cracking, and brittle (catastrophic) failure.
What is soaking in the heat treatment process?
Soaking is the second step of the heat treatment process. The metal is kept at an elevated temperature for a specific time (refer to the section on the stages of heat treatment).
Does annealing make metals stronger?
Annealing reduces internal stresses formed during prior forming and heat treatment processes. Reducing these stresses renders the metal more ductile and thus tougher, increasing its useful lifespan. Here, the flexural strength increases marginally.
Why does quenching harden steel?
Quenching freezes the microstructure achieved at elevated temperatures. Since this microstructure is usually less brittle and tougher than what is achieved at lower temperatures, the resulting metal tends to be more wear-resistant.
Does hardened steel rust?
Yes, hardened steel can still rust if subjected to adverse environmental conditions. However, in normal room temperature, the hardened steel takes slightly more time to rust when compared to soft steel.
What is the major disadvantage of hardened steel?
Hardened steel tends to be more brittle than its non-hardened counterpart. This could lead to cracks and catastrophic failure.




